Animal species: Adult, male white-tailed antsangy (Brachytarsomys albicauda)
Clinical history: The specimen was from an F1-generation colony (n=19 individuals) kept at a small zoological collection between 2020 and 2023. All antsangies had been bred at a breeding centre in the UK from animals imported from a Madagascan rescue centre. This animal had been found dead unexpectedly in its enclosure. Concurrent Hymenolepis nana infection was an ongoing issue for the juvenile antsangies within the colony, as previously reported by Archer et al. (2024). This was the only non-juvenile death in the group during the H. nana outbreak.
Organs: Liver and jejunum.
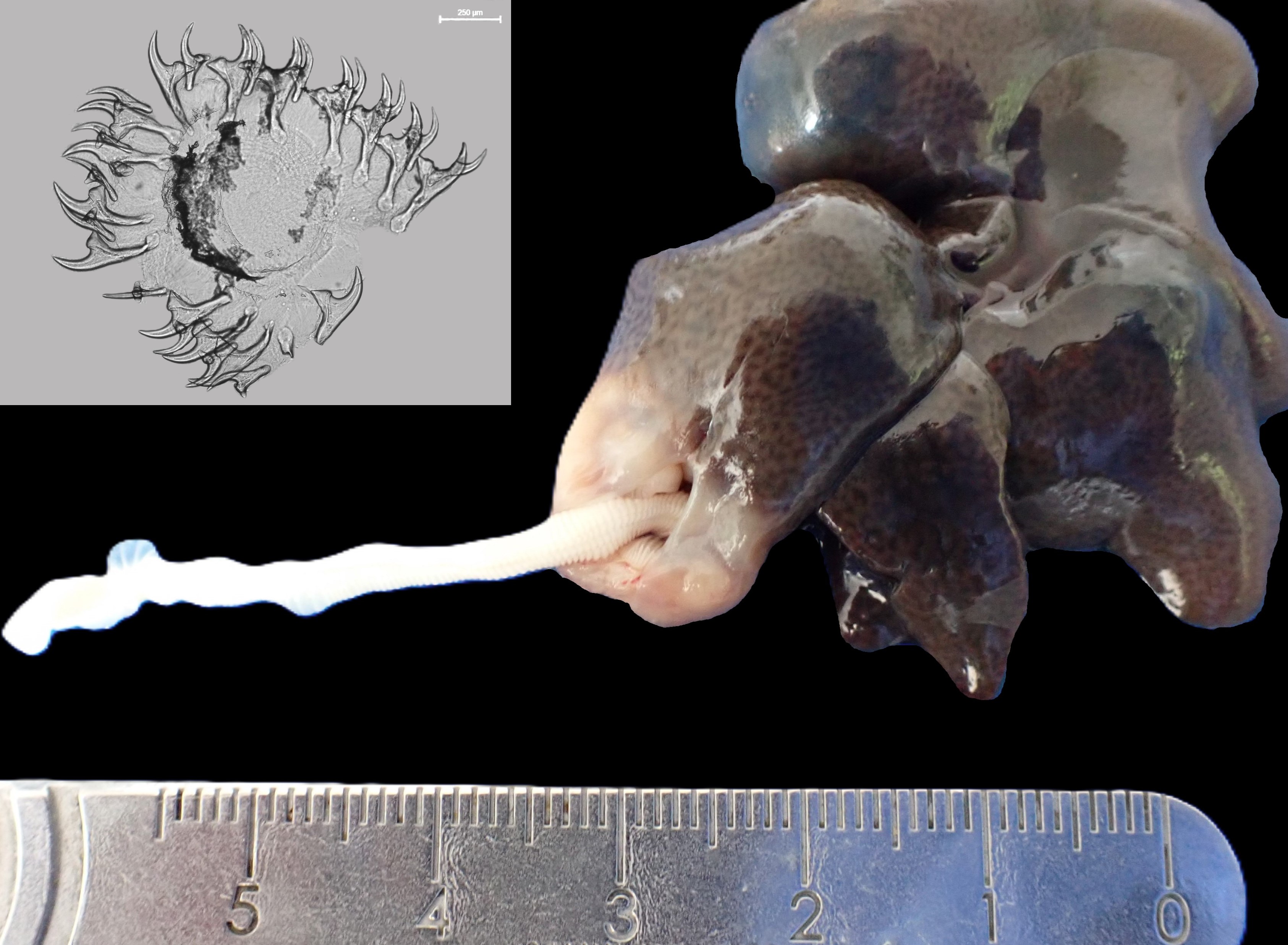
Macroscopical findings: On necropsy, the antsangy was in poor body condition. Two subcapsular cysts were identified in the liver. The right liver lobe cyst measured 16 x 8 x 7 mm and contained a 70 cm (long) x 4 mm (wide), off-white-to-light yellow, soft, regularly ridged pseudostrobila, while the left lobe cyst measured 12 x 12 x 10 mm and contained a 17 cm (long) x 4 mm (wide) off-white-to-light yellow, soft, regularly ridged pseudostrobila. Each pseudostrobila exhibited a single poorly defined scolex. Both cysts were thin-walled, fibrous and fluid filled, and compressed adjacent hepatic parenchyma. Additionally, a large multinodular mass (68 x 46 x 52 mm) was found in the proximal jejunum, associated with mucosal ulceration.
Histological findings: Both pseudostrobilae were not segmented, but each demonstrated a 10-20 µm thick, homogenous, acellular thick tegument, supported by a basement membrane, longitudinal subtegumental and transverse parenchymal muscle layers, and numerous 10 x 5-micron diameter, oval, clear structures containing central basophilic to eosinophilic amorphous material (calcareous corpuscles). No pseudocoeloms, digestive tracts or reproductive structures were identified in either parasite. The hepatic cysts exhibited oedematous fibrous capsules (100 micron thick) that were variably infiltrated by plasma cells, lymphocytes, few macrophages, and some eosinophils; with multifocal up to 250-micron diameter foci of dense clear acicular (cholesterol) clefts associated with regional areas of haemorrhage. In the jejunum, severe ulcerative and pyogranulomatous enteritis was present with sinus-like tracts that invaded and effaced the submucosa and muscle layers. These tracts contained coarse granular remnants of basophilic to eosinophilic amorphous material (suspect calcareous corpuscles) and occasional hypereosinophilic tegument-like fragments. Numerous dense Gram-positive rod-like bacterial colonies were present in the inflammatory infiltrate.
Complete faecal profile: Pure profuse isolate of Clostridium perfringens. No helminth eggs were detected on McMaster floatation.
Molecular identification of tapeworms:
Genomic DNA was extracted from both pseudostrobila. PCR and DNA sequencing for three genes (partial nuclear 28S rRNA, mitochondrial16S rRNA, and COX1) was conducted. Resultant data for both parasites matched published Genbank sequences as follows:
> 28S - Hydatigera taeniaeformis (96.14% - poor match)
> 16S - Hydatigera kamiyai (99.36% - better match)
> COX1 - Hydatigera kamiyai (99.52% - better match).
The reason for the lack of higher percentage matches with the 28S data was due to a lack of published data for this gene; only sequences for H. taeniaeformis are available from the genus Hydatigera. The resultant DNA sequences are available under GenBank accessions PQ479104–PQ479105 (28S), PQ463695–PQ463696 (16S), and PQ463655–PQ463656 (COX1).
Morphologic diagnosis:
Liver - Multifocal hepatic strobilocercosis.
Jejunum - Severe, focally extensive, chronic pyogranulomatous enteritis with intralesional cestode remnants and secondary Gram-positive rod-like bacteria.
Etiology: Hydatigera kamiyai
Name the disease: Strobilocercosis
Comment: This is the first documented case of Hydatigera kamiyai infection in a captive white-tailed antsangy. H. kamiyai belongs to the cestode genus Hydatigera and was recently described as a cryptic species (i.e., genetically distinct with little morphological variance) within the Hydatigera taeniaeformis complex. It has a northern Eurasian distribution, ranging from Europe to western Siberia, and China (Lavikainen et al., 2016; Miljević et al., 2023; Zhou et al., 2024). The intermediate hosts of H. kamiyai are primarily rodents from the subfamily Arvicolinae (e.g., voles, muskrats) and mice from the genus Apodemus (Lavikainen et al., 2016; Martini et al., 2022; Miljević et al., 2023). These rodents become infected through ingestion of cestode eggs in food or water contaminated by faeces of the definitive host, the domestic cat (Lavikainen et al., 2016; Zhou et al., 2024).
Since its description, H. kamiyai has been detected in additional hosts, such as the Plateau vole (Neodon fuscus; Zhou et al., 2024), a crowned sifaka (Propithecus coronatus; Verguin et al., 2024), and a bicoloured white-toothed shrew (Crocidura leucodon; Miljević et al., 2023). H. kamiyai has been speculated to pose a zoonotic risk to humans in contact with infected domestic cats (Miljević et al., 2023). This case expands the known host range for Hydatigera kamiyai, to now include a member of the Nesomyidae family of rodents. Death in this case was attributable to bacterial septicaemia (C. perfringens) secondary to the significant enteric ulceration caused by the invasive parasites.
Educational note: A strobilocercus is a specific larval/metacestode stage of some cestodes, particularly in the family Taeniidae, that is characterised by its segmented-like structure that grossly resembles an immature tapeworm. Histologically, however, a strobilocercus lacks a digestive tract and reproductive structures.
Contributors: Andrew F. Rich BVSc DiplECVP AFHEA MRCVS (International Zoo Veterinary Group (IZVG) Pathology, West Yorkshire, UK – email: a.rich@izvg.co.uk), Claire Griffin (Sequencing Facility, Science Innovation Platforms, Natural History Museum, UK), Karen R. Archer BVSc BSc (Hons) CertAVP (ZooMed) MRCVS (International Zoo Veterinary Group, West Yorkshire, UK), Dr Tim Littlewood BSc (Hons) PhD DSc and Dr Andrea Waeschenbach BSc PhD (Science, Natural History Museum, London, UK).
References:
Archer KR, Waeschenbach A, Griffin C, Payne IL, Houston J, Littlewood DT, Rich AF. Fatal Hymenolepis nana-associated visceral larva migrans in captive juvenile white-tailed antsangies (Brachytarsomys albicauda). Journal of Comparative Pathology. 2024;212:32-41.
Lavikainen A, Iwaki T, Haukisalmi V, Konyaev SV, Casiraghi M, Dokuchaev NE, Galimberti A, Halajian A, Henttonen H, Ichikawa-Seki M, Itagaki T. Reappraisal of Hydatigera taeniaeformis (Batsch, 1786) (Cestoda: Taeniidae) sensu lato with description of Hydatigera kamiyai n. sp. International Journal for Parasitology. 2016;46(5-6):361-74.
Martini M, Dumendiak S, Gagliardo A, Ragazzini F, La Rosa L, Giunchi D, Thielen F, Romig T, Massolo A, Wassermann M. Echinococcus multilocularis and other taeniid metacestodes of muskrats in Luxembourg: Prevalence, risk factors, parasite reproduction, and genetic diversity. Pathogens. 2022;11(12):1414.
Miljević M, Rajičić M, Umhang G, Bajić B, Bjelić Čabrilo O, Budinski I, Blagojević J. Cryptic species Hydatigera kamiyai and other taeniid metacestodes in the populations of small mammals in Serbia. Parasites & Vectors. 2023;16(1):250.
Verguin C Brunet J Ferte H, Gravet A, Gay A, Ferreira X, Dedeyan A, Debar J, Lemberger K, Lefaux B, Quintard B. Hemiparesis management and cestode infection (Hydatigera kamiyai) in a crowned sifaka (Propithecus coronatus). 2024 Joint AAZV-EAZWV Conference Proceedings, Toronto, Canada.
Zhou G, Zhang H, Chen W, Li Z, Zhang X, Fu Y. Morphological observation, molecular identification and evolutionary analysis of Hydatigera kamiyai found in Neodon fuscus from the Qinghai-Tibetan plateau. Infection, Genetics and Evolution. 2024;123:105629.